 |

X-ray Photelectron Spectroscopy (XPS) is used in research, development and manufacturing. This technique is able to obtain the chemical composition of various material surfaces up to 1 nm depth. It is possible to find out if this material is superficially oxidised, if it contains iron, or carbon, etc.
Most of the elements can be detected except hydrogen. Our main interest is to find out the composition i.e. the atomic percentage of each of the components. Using these characterisation means implies defined rules. Let me explain for you the principle and the various steps of such an analysis.  XPS analysis basics. XPS analysis basics.
Let's refresh our knowledge together:
Each electron is carrying an ID card with its address. The goal is to catch them in order to find out from which atom they are coming. To be able to free them from the nucleus attractive force we excite them by exposure to X-ray bombardment. This kind of light is bringing them enough energy to free them from the nucleus. Once they have been freed up some on them travel through the matter and reach its surface. Electrons which carry enough energy at this point, may leave the solid matter for the surrounding vacuum. Once they are in the vacuum, they are collected by and electron analyser. Furthermore they are classified according to their ID cards. The information we are interested in is the so-called binding energy which they had before leaving the atom. All what we do is counting these electrons versus their binding energy and we obtain a spectrum looking like the one shown below: 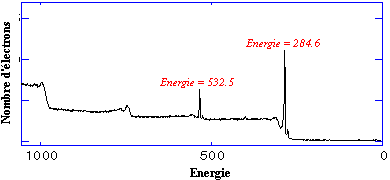 Electron count plot versus energy (energy spectrum) If we observe the spectrum, we see two main peaks at 284.6 and 532.5 energy counts. The unit used for counting energy is electronvolt - abbreviated as eV. - We simply mention that there are peaks at 284.6 eV and 532.5 eV. Each energy matches a specific atom type, e.g. 284.6 eV matches carbon and 532.5 matches oxygen. From this we can conclude that this specimen contains carbon and oxygen. Each peak area is proportional to the number of atoms being present in the studied element. By calculating the respective contribution of each area we obtain the specimen chemical composition, for example: 25% Oxygen and 75% Carbon. In other words, among 100 atoms present at the surface of the material, 75 are carbon atoms. By studying the energy of this carbon peak, it is possible to find out if the surface of this material corresponds to C-O or C=O chemical form. We can interpret the experiment by the mean of result modelling. For more information, select a higher level of knowledge: Level 2.  A step-by-step XPS analysis. A step-by-step XPS analysis.
In a first step it is necessary to prepare of the specimen. The size may vary from a few millimetres to a few centimetres, depending partly on the instrument but mainly on the technology being used.
The specimen is then introduced in the first chamber (sample preparation chamber). This chamber is then pumped by high vacuum pumps (also called secondary vacuum pumps) down to a vacuum below 10-7 mbar. When the proper vacuum has been achieved, the specimen is transferred into the analysis chamber and the XPS experiment may begin. The analysis chamber vacuum ranges from 10-8 mbar to 10-11 mbar. The next illustration shows a schematic drawing of the XPS spectrum acquisition principle. For clarity, the drawing scale has been changed.
 Conclusion. Conclusion.
Photoelectron spectroscopy (XPS) is a dedicated surface characterisation spectroscopy. It reveals which chemical elements are present at the surface, for example carbon and oxygen; it informs us about the chemical bound nature which exists between these elements. An appropriate data processing leads to the specimen elemental composition.
|