 |

X-ray Photoelectron Spectroscopy known as XPS or ESCA (Electron Spectroscopy for Chemical Analysis) has been developed from the Fifties by Professor K. Siegbahn. The Physics Nobel Prize awarded his work in 1981. The most interesting thing with this technique is its ability to measure binding energy variations resulting from their chemical environment. For the past 20 years, this type of spectrometry emerged as a key tool in surface analysis, mainly because of two major features:
This page reviews the photoemission principle and the technical data of XPS spectrometry. 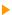 Photoemission principle. Photoemission principle.
By absorbing a photon, an atom gains an energy amount equal to h?. It then releases an electron to regain its original stable energy state. The released electron retains all the energy from the striking photon. It can then escape from the atom, and even further from matter and kinetic energy keeps it moving. With XPS, incident photons usually carry an energy ranging from 1 to 2 KeV.
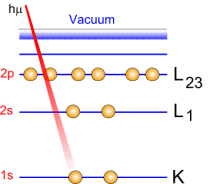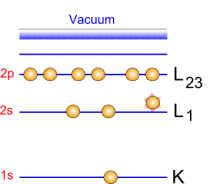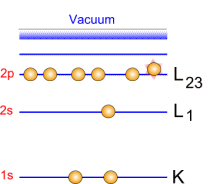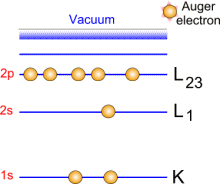 Photoemission principle. For example, by usual X-ray sources, magnesium and aluminium emit at 1253.6 and 1486.6 eV respectively. The relative high level of the incident energy causes the matter to release and electron from an atom internal shell. Consequently, there will be some atoms lacking electrons in the internal shells from which photoelectrons have been released. To recover from this ionised state the atom can emit another photon (fluorescence) or undergo an Auger transition. The principle of the conservation of energy allows us to write the energy balance equation, valid for the absorption of a photon carrying an energy of hn hn: X-ray beam incident energy E cinétique: Electron kinetic energy when leaving the specimen. E liaison: Electron binding energy inside the atom.  Photoelectron energy. Photoelectron energy.
We shall remember that a photoelectron carrying enough energy can leave the material. In order to determine its binding energy within the matter we must first measure it's energy when it has left the matter.
A lens system focuses the electron beam into a hemispherical analyser. This analyser consists of two plates carrying a potential. When the electron is entering the analyser it undergoes and electrical field which forces it to travel along the trajectory defined by the following equation: According to this equation, a given electrical field or a given U potential corresponds to a given kinetic energy. It means that the hemispherical analyser behaves like an energy filter. 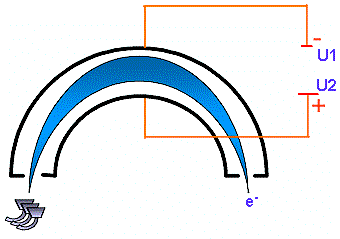 Principle of kinetic energy determination |